Page 64 - วารสารกรมการแพทย์แผนไทยฯ ปีที่ 11 ฉบับที่ 3
P. 64
Journal of Thai Traditional & Alternative Medicine Vol. 11 No. 3 September-December 2013 273
227 nm, EI-MS: m/z (relative intensity, %); °—¥À¬“∫‡∂“«—≈¬å‡ª√’¬ß¥â«¬ 50% ‡Õ∑“πÕ≈ ¥â«¬
[C H O ] 279.47 [M+Na] (65). genistein 7- «‘∏’‚§√¡“‚∑°√“øï ¡√√∂π– Ÿßæ∫«à“¡’Õߧåª√–°Õ∫
11 12 7
O-α-rhamnosyl(1→6)-β-glucopyranoside (2): ∑“߇§¡’√«¡ª√–¡“≥ 13 ™π‘¥ ‚¥¬æ∫ genistein
λ = 261 nm, EI-MS : m/z (relative inten- 7-O-α-rhamnosyl (1→6)-β-glucopyranoside (2)
max
sity, %) [C H O ], 601.55 [M+Na] (60), ‡ªìπÕߧåª√–°Õ∫À≈—°∑’Ë retention time ‡∑à“°—∫ 8.75
27 31 14
579.59(40), 433.63 (30), 271.58 (100) ‚¥¬ Ÿμ√ π“∑’ ·≈– piscidic acid (1) ‡ªìπÕߧåª√–°Õ∫√Õß∑’Ë
‚§√ß √â“ß∑“߇§¡’¢Õß “√∑—Èß Õß™π‘¥· ¥ß„π√Ÿª∑’Ë 3 retention time ‡∑à“°—∫ 1.45 π“∑’ (√Ÿª∑’Ë 4) º≈
®“°º≈°“√«‘‡§√“–ÀåÕߧåª√–°Õ∫∑“߇§¡’¢Õß “√ °“√»÷°…“ƒ∑∏‘Ϭ—∫¬—È߇Õπ‰´¡å COX æ∫«à“ 1 ‰¡à¡’
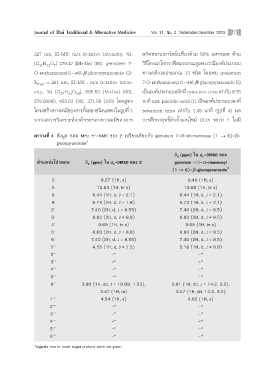
1
μ“√“ß∑’Ë 4 ¢âÕ¡Ÿ≈ 500 MHz H -NMR ¢Õß 2 ‡ª√’¬∫‡∑’¬∫°—∫ genistein 7-O-α-rhamnosy (1 → 6)-β-
5
glucopyranoside
δ (ppm) „π d -DMSO ¢Õß
H 6
μ”·Àπàß‚ª√μÕπ δ (ppm) „π d -DMSO ¢Õß 2 genistein 7-O-α-rhamnosyl
H
6
5
(1 → 6)-β-glucopyranoside
2 8.27 (1H, s) 8.40 (1H, s)
5 12.83 (1H, br s) 12.88 (1H, br s)
6 6.44 (1H, d, J = 2.1) 6.44 (1H, d, J = 2.1)
8 6.73 (1H, d, J = 1.8) 6.73 (1H, d, J = 2.1)
2' 7.40 (2H, d, J = 8.55) 7.40 (2H, d, J = 8.5)
3' 6.83 (2H, d, J = 8.8) 6.83 (2H, d, J = 8.5)
4' 9.65 (1H, br s) 9.65 (1H, br s)
5' 6.83 (2H, d, J = 8.8) 6.83 (2H, d, J = 8.5)
6' 7.40 (2H, d, J = 8.55) 7.40 (2H, d, J = 8.5)
1'' 4.55 (1H, d, J = 7.3) 5.18 (1H, d, J = 6.9)
2'' -* -*
3'' -* -*
4'' -* -*
5'' -* -*
6'' 3.90 (1H, dd, J = 10.99, 1.53), 3.91 (1H, dd, J = 14.2, 3.2),
3.47 (1H, m) 3.47 (1H, dd, 14.2, 3.2)
1''' 4.54 (1H, s) 4.62 (1H, s)
2''' -* -*
3''' -* -*
4''' -* -*
5''' -* -*
6''' -* -*
*signals due to most sugar protons were not given